

The value of
equilibrium constant can be calculated of we know the…..of reactants and
produce in equilibrium mixture.
a) concentration
b) suspension
d) moles
d) compound
The equilibrium constant can be used to predict the….and extent of chemical reaction.
a) reactant
b) products
c) direction
d) concentration
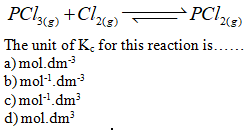
When the ratio
of [product]/[Reactants] is less than Kc and system is not at
equilibrium then more…… is required to reach the equilibrium.
a) products
b) reactants
c) heat
d) None of these
When the ratio of [products]/[Reactants] is greater than Kc and system is not at equilibrium then more……….is required to such equilibrium.
a) reactants
b) product
c) heat
d) None of these
In Birkland-Edge process, nitrogen produces………., which is an important chemical compound.
a) Sulphuric acid
b) Ammonia
c) Nitric acid
d) Oxygen