


Hydrochloric
acid gas combines with ammonia gas resulting in white smoke of solid………
a) hydrogen chloride
b) ammonium chloride
c) both a & b
d) none of these
When an acid molecule lose a proton form…..
a) conjugate acid
b) conjugate base
c) both a & b
d) none of these
Explanation:when a base takes a proton then it forms positively charge species which can act as an acid and is called as conjugate acid of the corresponding base e.g
.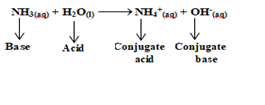
In
this reaction ammonium ion (NH4+)
is called a conjugate acid of ammonia (NH3).
A
species which accept proton, form ………of the corresponding acid.
a) conjugate acid
b) conjugate base
c) both a & b
d)
none of these
Explanation:When an acid gives a proton ( H+) it forms negatively charged species which can accept proton and act as a base is called conjugate base of the corresponding acid e.g
.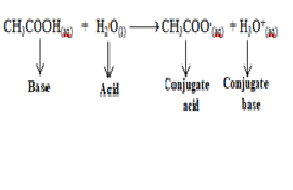
When
an acidic acid (CH3COOH )
loses a proton it forms CH3COO- ion which can take a
proton and act as a base and thus CH3COO- is called
conjugate base of the acid (CH3COOH ).
Concept of acids and bases :-
A third classification of acids and bases was introduced in………
a) 1992
b) 1923
c) 1924
d) 1925