

Electrical-conductivity experiment have shown that pure………is an extremely weak electrolyte.
a) water
b) salt
c) ammonia
d) ammonium ion
Water undergoes self-ionization to a very.…… extent.
a) large
b) small
c) both a and b
d) none of these
Pure water contains………number of H+ and OH-.
a) unequal
b) equal
c) both a and b
d) none of these
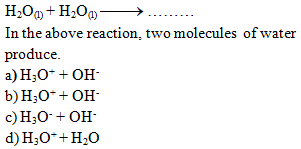
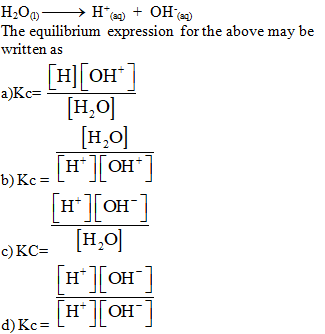
……is
called water dissociation constant.
a) Kw
b)
Kc
c) K
d) KC