

The general molecular formula of alkane is…
a) CnH2n
b) CnH2n+1
c) CnH2n+2
d) CH2n+1
In “CnH2n+2” n stands for the number of……… atom in alkane.
a) oxygen
b) carbon
c) sulphur
d) nitrogen
…………is an example of alkane.
a) ethane
b) ethene
c) ethyne
d) cyclobutene
Propane is differed from ethane by one carbon and…………hydrogen atoms.
a) 12
b) 8
c) 2
d) 4
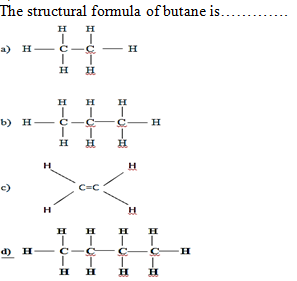
For naming of
alkane, 1st we count the number of…..atoms in the formula of alkane.
a) oxygen
b) sulphur
c) carbon
d) nitrogen