

Soaps are the sodium salts of the ………..
a) protein
b) fatty acid
c) carbohydrate
d) nucleic acid
When soap is added to hard water it is ionized into …………….
a) sodium ion
b) stearate ion
c) both a & b
d) none of these
The ………… ions are present in water.
a)Ca+2
b) Mg+2
c) Na+2
d) both a & b
The ions react with soap and produce an insoluble precipitate of calcium & magnesium steartes, which is called ………..
a) silicate
b) Zeolite
c) scum
d) none of these
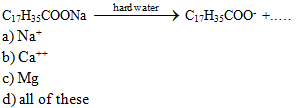
The chemical
formula of sodium stearate is…...
a) Ca++
b) C17H35COO
c) C17H35COONa
d) (C17H35COO)2Ca