

According to Arrhenius………. is a chemical substance that dissociate in aqueous solution to give hydroxide ion.
a) acid
b) base
c) both a & b
d) None of these
Most of the bases dissociate in a solution to release……….
a) O-H
b) H+
c) H3Od) NH4
Other bases are substances that react with ……….to remove a hydrogen ion leaving hydroxide ions in the solution.
a) ester
b) phenol
c) water
d) ether
The substances such as………are bases.
a) NaOH , KOH
b) Mg(OH)2, Ca(OH)2
c) NH4OH
d) All of these
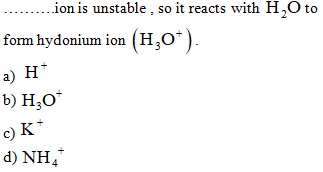
When……….ionize
in aqueous solutions to produce OH+ions.
a) NaOH
b) NH4OH
c) Ca2+
d) both a & b