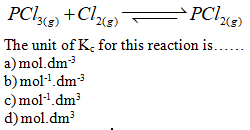The equilibrium
constant (Kc) is …….
a) the sum of two reactants
b) the difference of the two rate constant
c) the product of the two rate constant
d) the ratio of the two rate constant
When the value
of Kc is very small it shows that……….
a) reaction will go in forward direction
b) reaction will go in reverse direction
c) reaction is at equilibrium
d) equilibrium will never established
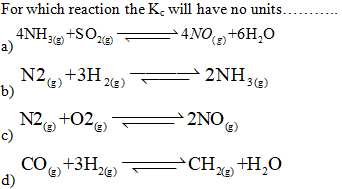
When the value
of Kc is very large it shows that………..
a) reaction is at equilibrium
b) equilibrium will never be achieved
c) reaction will move in reverse direction
d) reaction will move in forward direction
Active masses means………….
a) the total mass of reactants
b) the total mass of products
c) the total mass of reactants
d) concentration of reactant and products in moles per dm3 in dilute solution.