

Angular momentum of an electron is an integral multiple of …………..
a) hƲ
b) mvr
c) 2h/π
d) h/2π
mvr = …….
a) nh/2π
b) 2h/nπ
c) nh/2
d) h/2nπ
mvr = nh/2π , h denotes……
a) spring constant
b) Lenz’s constant
c) plank’s constant
d) none of these
The value of Plank’s constant “h” is...……….
a) 6.62 × 10-34j.s
b) 7.72 × 10-34j.s
c) 8.82 × 10-34j.s
d) 9.93 × 10-34j.s
Energy levels are denoted ………..
a) K
b) M
c) L
d) all of these
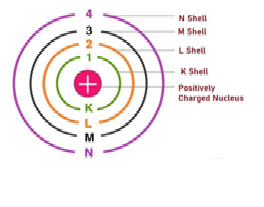
The orbit having least energy is ……..
a) K
b) M
c) L
d) N