

Single covalent bond exists in …………
a) H2
b) F2
c) Cl2
d) all of these
Chlorine attains the electronic configuration by sharing of one electron.
a) He
b) Ne
c) Ar
d) Kr
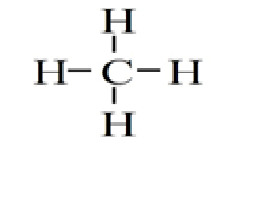
Methane is an example of …………… covalent bond
a) single
b) double
c) triple
d) none of these
The bond in which two atoms share two electron each called
a) single covalent bond
b) triple covalent bond
c) double covalent bond
d) ionic bond
A double bond is denoted by a………lines.
a) single
b) double
c) triple
d) none of these
Oxygen attains the electronic configuration of which element by sharing of two electrons.
a) He
b) Ne
c) Ar
d) Kr