

If the electronegativity difference is less than 1.7 the bond will be ………
a) ionic
b) covalent
c) metallic
d) a & b both
The covalent bond in which only one atom donates the shared pair of electron is called…
a) Coordinate covalent bond
b) ionic bond
c) covalent bond
d) metallic bond
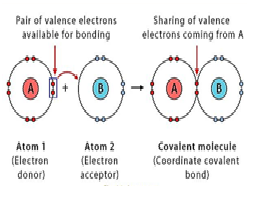
Coordinate covalent bond is also called ……
a) dative bond
b) single covalent bond
c) double covalent bond
d) triple covalent bond
The atom which donates the shared pair of electrons is called …….
a) donor
b) acceptor
c) a & b both
d) none of these
The atom which accepts the shared pair of electrons is called.…….
a) donor
b)acceptor
c) a & b both
d) none of these
Dative bond is represented by.……….
a) single line
b) double line
c) triple line
d)arrow