

Which relation is true ?
a) A = Z - n
b) A = Z + n
c) Z = A + n
d) n = Z + A
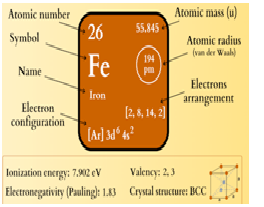
Iron has atomic number 26 and mass number 56.
Number of neutrons in it are………..
a) 29
b) 27
c)
30
d) 28
Isotopes are the elements have same number of protons but different
number of……..
a) Electrons
b) Neutrons
c) a & b both
d) none of these
Carbon has ………. isotopes.
a) 2
b) 3
c) 4
d) 7
A substance whose atoms have the same atomic number is called…………
a) compound
b) element
c) mixture
d) substance
The sum of protons and neutrons in the nucleus of an atom is called………
a) atomic Number
b) mass number
c) formula Mass
d) atomic Mass