

The sub shell ‘s’ stands for ……………
a) sharp
b) sweet
c) smart
d) all of these
Sodium has electronic distribution such as
a) 1s2,2s2,2p6,3s1
b) 1s1,2s2,2p6,3s2
c) 1s1,2s1,2p6,3s3
d) 1s2,2s1,2p6,3s2
The sub shell ‘p’ stands for ……………
a) principal
b) partly
c) principle
d) potential
“Cl” has an electronic configuration…………
a) 1s1,2s3,2p2,3s2,3p3
b) 1s1,2s3,2p4,3s2,3p3
c) 1s1,2s3,2p2,3s2,3p3
d) 1s2,2s2,2p6,3s2,3p5
The sub shell ‘d’ stands for ……………
a) degraded
b) diffused
c) distributive
d) all of these
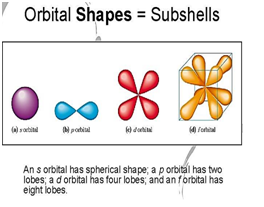
“s” sub shell carries ……… orbitals.
a) 1
b) 3
c) 2
d) 5