

Every system in universe tends to lower energy to attain ………..
a) stability
b) instability
c) a & b both
d) none of these
Explanation:Energy and stability are inversely proportional to each other . lower is the energy of molecule more will be stability of the molecule.
Atoms have tendency to ………their energy.
a) decrease
b) increase
c) a & b both
d) all of these
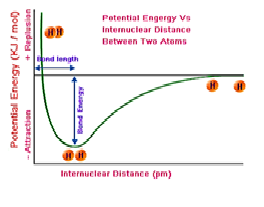
The energy of an isolated hydrogen atoms is …… than the two bonded hydrogen atoms.
a) lower
b) higher
c) same
d) none of these
The concept which explain the chemical bonding is ……….
a) valance concept
b) orbital concept
c) a & b both
d) Lewis concept
The valence concept is also called :
a) molecular orbital concept
b) electronic theory of valance
c) valence bond concept
d) none of these
Explanation:The Electronic theory of valency states that every element has a tendency to attain electronic configuration of the nearest inert gas, as it is the most stable configuration called stable octet, which consists of 8 electrons or a duplet consisting 2 electrons and the number of electrons it accepts or donates is its valency.
In which year electronic theory of valance was given ?
a) 1916
b) 1919
c) 1913
d) 1923