

Electrons are gained in …………
a) oxidation
b) reduction
c) a & b both
d) none of these
The addition of oxygen to a substance is called ……….
a) catenation
b) combustion
c) oxidation
d) reduction
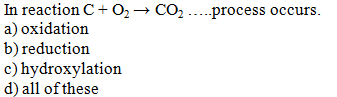
The removal of hydrogen from a substance is
called ……….
a) oxidation
b) reduction
c) a & b both
d) none of these
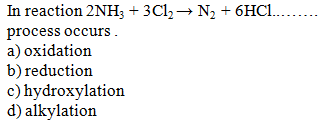
Explanation:Removal of hydrogen is called reduction. In
this reaction hydrogen is removed from ammonia, so reduction is involved in
this reaction.
Fe2+ ……to Fe3+.
a) reduces
b) oxidizes
c) a & b both
d) none of these
Explanation:Electrons are lost in oxidation so above reaction involves oxidation.