Atomic theory was presented in………..
a) 1810
b) 1832
c) 1808
d) 1884
According to atomic theory “ Atom is…… particle”
a) divisible
b) huge
c) indivisible
d) none of these
Explanation:It was thought that atom was indivisible particle. Researchers have just shown how a single atom can be split into its two halves, pulled apart and put back together again. While the word "atom" literally means "indivisible," the laws of quantum mechanics allow dividing atoms -- similarly to light rays -- and reuniting them.
According to atomic theory “Atoms of different elements are……in their properties”.
a) same
b) different
c) inert
d) none of these
Rutherford performed an experiment in……
a) 1913
b) 1914
c) 1911
d) 1917

Rutherford performed an experiment to determine………..
a) structure of atom
b) energy of electron
c) diameter of an atom
d) radius of electron
Rutherford used thin foil of………..
a) o.oo4 cm
b) 0.00004 cm
c) o.oo3 com
d) 0.00003 cm
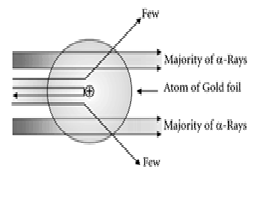
Explanation:Rutherford designed an experiment for this. In this
experiment, fast moving alpha (α)-particles were made to fall on a thin
gold foil. He selected a gold foil because he wanted as thin a layer as
possible. ... Since they have a mass of 4µ, the fast-moving α-particles have a
considerable amount of energy.