

Stability of noble gases is due to having ………outermost shells.
a) partially filled electrons
b) filled
c) empty shells
d) none of these
All the noble gases have eight electrons in their valence shell except ……..
a) neon
b) argon
c) krypton
d) helium
Explanation:Helium has two electrons in its valence shell and also has atomic number of two.
A shell with eight electrons is called ……..
a) duplet
b) octet
c) a & b both
d) none of these
The overlapping of orbitals can be ……….
a) endwise
b) sidewise
c) a & b both
d) none of these
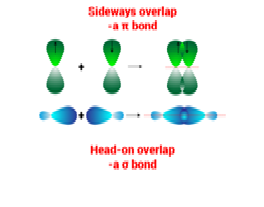
Endwise overlapping produce ……….
a) sigma bond
b) pi-bond
c) a & b both
d) no bond
Explanation:In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
Sidewise overlapping produce ……….
a) sigma bond
b) pi-bond
c) a & b both
d) no bond
Explanation:In chemistry, pi bonds are covalent chemical bonds, in each of which two lobes of an orbital overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei.