

Types of bonds are ………
a) 2
b) 5
c) 3
d)4
Explanation:There are four types of bonds. Ionic bond , Covalent bond , Dative bond and Metallic bond.
Type of bond is …………
a) ionic bond
b) covalent bond
c) metallic bond
d) all of these
The chemical bond which is formed due to complete transfer of electron is called.………
a) metallic bond
b) ionic bond
c) dative covalent bond
d) covalent bond
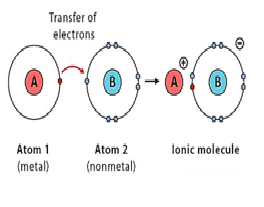
Ionic bond is also called..……..
a) electrovalent bond
b) valent bond
c) molecular bond
d) metallic bond
Ionic bond is always formed between……….. and non metals.
a) non metals
b) metals
c) metalloids
d) none of these
Metals always ……..electrons.
a) lose
b) gain
c) share
d) all of these
Explanation:Metals generally lose electrons to complete their octet and non-metals gain electrons to complete their octet. Metal atoms lose electrons from their outer shell when they form ions: the ions are positive, because they have more protons than electrons.