

A solution of known concentration is called
a) dilute solution
b) concentrated solution
c) saturated solution
d) standard solution
Explanation:In analytical chemistry, a standard solution is a solution containing a precisely known concentration of an element or a substance. A known mass of solute is dissolved to make a specific volume. It is prepared using a standard substance, such as a primary standard.
Units to express the concentration of solutions are ……..
a) two
b) four
c) three
d) many
Explanation:A number of units are present to express the concentration of solution such as percentage composition, molarity, molality, formality, normality, ppm, ppb, mole fraction.
Number of parts of solute in 100 parts of the solution is known as ………
a) parts per million
b) molarity
c) percentage composition
d) molality
Number of grams by mass of solute present in 100g by mass of solution is called ……..
a) %age m/V
b) %age m/m
c) %age V/m
d) %age V/V
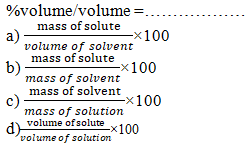
10% m/m solution of sugar means ……..
a) 10g sugar in 110g solvent
b) 100g sugar in 10g solvent
c) 10g sugar in 90g solvent
d) 90g sugar in 10g solvent