

Which of the following reaction takes place during the extraction of iron from iron oxide by heating with coke?
a) burning
b) displacement
c) double-displacement
d) addition
A chemical reaction in two atoms or group of atoms exchange places and form new compounds is called…….reaction.
a) burning
b) displacement
c) double-displacement
d) addition
Explanation:A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. The general form of a double-replacement (also called double-displacement) reaction is:AB+CD→AD+CB
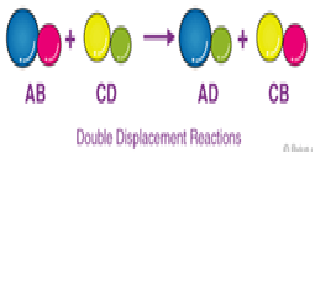
Neutralization
reaction between and acid and base is………
a) burning
b) displacement
c) double-displacement
d) addition
Explanation:neutralization or neutralisation is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution.
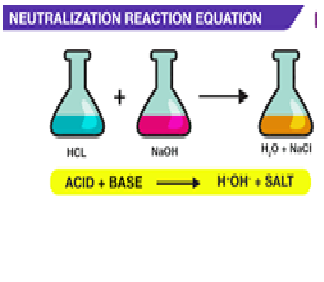
When silver
nitrate solution is added to sodium chloride solution, they produce silver
chloride and sodium …….
a) chloride
b) nitrate
c) chlorate
d) nitrite
When silver nitrate solution is added to sodium chloride solution, they produce silver chloride and sodium nitrate. Which type of reaction is involved?
a) combustion
b) decomposition
c) double-displacement
d) addition
Which of the following is/are produced during combustion?
a) CO2
b) heat
c) light
d) all of these