

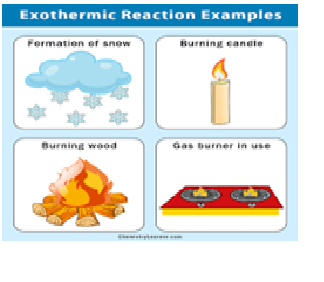
Burning or combustion is an example of …….reaction.
a) decomposition
b) displacement
c) endothermic
d) exothermic
Which of the following is/are exothermic reaction?
a) CH4(g) + 2O2 ----à 2H2O(l) + heat + light
b) CH4(g) + 2O2(g)---à CO2(g) + 2H2O(l) + heat+ light
c) 2H2(g) + O2(g) ---à 2H2O(g) + heat + light
d) all of these
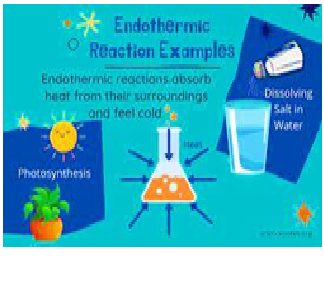
A chemical
reaction that absorbs energy from their surroundings is called……..reaction.
a) decomposition
b) displacement
c) endothermic
d) exothermic
Explanation:an endothermic process is any process with an increase in the enthalpy H of the system. In such a process, a closed system usually absorbs thermal energy from its surroundings, which is heat transfer into the system.
Endothermic is
basically…….word.
a) Greek
b) Russian
c) English
d) Dutch
“Endo” is a Greek word which means?
a) to absorb
b) to evolve
c) release
d) to attract
Which lime stone is heated at 1000 C, it decomposes. What type of reaction this is?
a) addition
b) displacement
c) endothermic
d) exothermic