

Which of the following reaction/s show/s endothermic reaction/s?
a) CaCO3(s) + heat -------àCaO(s) + CO2(g)
b) 2H2O2(l) +electricity-----à 2H2O(l) + O2(g)
c) 2H2(g) + O2(g) ---à 2H2O(g) + heat + light
d) both a and b
All endothermic reactions need a continuous source of ………
a) mass
b) gas
c) energy
d) air
What is required for exothermic reactions in the beginning of the process?
a) mass
b) gas
c) energy
d) air
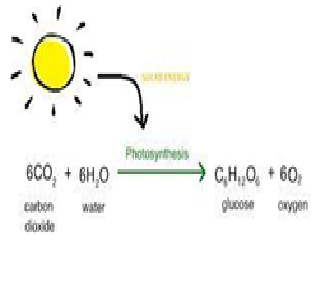
Photosynthesis needs sunlight energy. What type of this reaction is?
a) addition
b) displacement
c) endothermic
d) exothermic
Explanation:Photosynthesis is an endothermic reaction. This means it cannot occur without energy (from the Sun). The light required is absorbed by a green pigment called chlorophyll in the leaves.
Ozone (O3)
absorbs UV rays in the upper atmosphere and converts into O2. What
type of this reaction is?
a) addition
b) displacement
c) endothermic
d) exothermic
Explanation:The ozone splitting is an exothermic reaction. This means that ozone splitting results in heat which causes an increase in atmospheric temperature.
Which of the following protects living things on the earth from UV radiation coming from the sun?
a) CO2
b) O2
c) O3
d) all of these