

How many periods are there in the periodic table?
a) 7
b) 8
c) 10
d) 18
Which of the following property/ies of an atom is shown by the period number?
a) valence electron
b) valence shell number
c) number of shells
d) all of these
Explanation:The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. Electrons in outer shells have higher average energy and travel farther from the nucleus than those in inner shells.
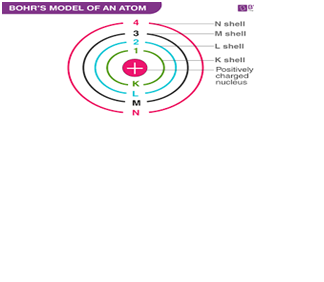
Elements in the
first shell have…… electronic shell/s.
a) 1
b) 2
c) 7
d) 8
How many shells are present in the element of the second period?
a) 1
b) 2
c) 3
d) 4
How many shells are present in the element of the third period of the periodic table?
a) 1
b) 2
c) 3
d) 4
How many shells are present in the element of the fourth period?
a) 1
b) 2
c) 3
d) 4
2 electron
by at 2025-02-10 06:35:35