Which of the following elements are called main group elements?
a) group A
b) Group B
c) both a and b
d) none of these
Explanation:Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
Which of the following elements are called transition elements?
a) group A
b) Group B
c) both a and b
d) none of these
Explanation:Group B metals are referred to as transition metals. They are located in the middle of the periodic table between Group IIA and Group IIIA.
First 18 elements present in which of the following group/s?
a) group A
b) Group B
c) both a and b
d) none of these
What is the common name of elements present in group IA?
a) the alkali metals
b) the alkaline earth metals
c) the halogens
d) the noble gases
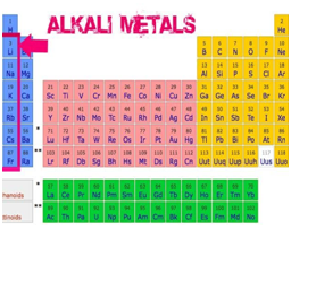
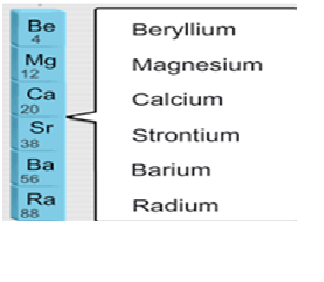
What is the
common name of elements present in group IIA?
a) the alkali metals
b) the alkaline earth metals
c) the halogens
d)
the noble gases
Explanation:The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium
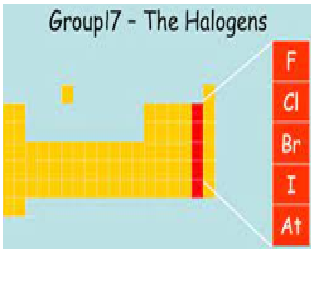
What is the
common name of elements present in group VIIA?
a) the alkali metals
b) the alkaline earth metals
c) the halogens
d) the noble gases
Explanation:The halogens are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).