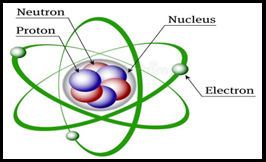
- There are three
sub-particles of atoms (Proton, Neutron, and Electron). These are
also called fundamental particles.
-
The center of the atom is called ‘Nucleus’.
- Protons and neutrons
is the part of nucleus.
- Electron always in constant motion around the nucleus.
- Electron has negative charge.
- Proton has positive charge.
- Neutron has no charge.
- Electrons revolve around the nucleus in particular paths
called orbits or shells.
- Atom is neutral particle. (The number of proton just equal to the
number of electron).
-
The number of protons in the nucleus or the no of electron of
an atom is called the atomic number. Represented by ‘Z’.
-
The total number of proton and neutron in nucleus is called Mass
number. Represented by ‘A’.
-
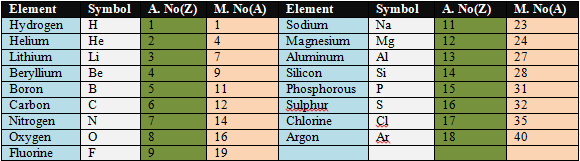
Atomic Number and Mass Number of First 18 elements.
- The formula
of electronic configuration is 2n2.
- Shell or energy level represented by K(1st )=2,L(2nd
)=8, M(3rd )=18,N(4th)=32
- Atomic no and Mass no written method 11Na23, 6C12.
- The electron in the highest shell is involved in bonding
and they are called valence electron.
- An ion is formed when an atom loses or gains
an electron during a chemical reaction.
- When an atom loses electron it form positive ion.
- The Positive ion is called ‘cation’.
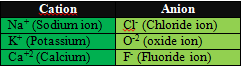
- When an atom gains electron
it forms a negative ion.
- The negative ion is called ‘anion’.
- Some element has two or more different atoms. These atoms have same
atomic number but different mass number is called isotopes.
( 1H1, 2H1, 3H1)
These have the same number of protons in each atom but the neutron
number can vary…
a) Isotopes
b) Molecules
c) Ions
d) Isobars
a
An atom of carbon contains 6 protons and 6 neutrons, its mass number will be…………….
a) 12
b) 6
c) 18
d) 24
a
The center or core of an atom is called………..
a) Orbital
b) Nucleus
c) Proton
d) Neutron
b
An element has seven electrons in its valance shell. Its valancy is………..
a) 7
b) 0
c) -1
d) 8
c
Atoms are composed of … types of particles.
a) two
b) three
c) four
d) five
b




