

Structure of an ATOM
Atoms consist
of an extremely small, positively charged nucleus surrounded by a cloud of
negatively charged electrons. Although typically the nucleus is less than one
ten-thousandth the size of the atom, the nucleus contains more that 99.9% of
the mass of the atom.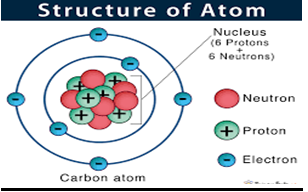
The
matter is made up of tiny particles called
a) atoms
b)
molecules
c) compound
d) mixture
Which of the following is pure matter made of same type of atoms?
a) elements
b) molecules
c) compound
d) mixture
Explanation:A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction.
Which of the following is the unit of matter?
a) atoms
b) molecules
c) compound
d) mixture
Which of the following is/are element/s?
a) gold
b) copper
c) water
d) both a and b
Which of the following is/are NOT element/s?
a) alcohol
b) salt
c) water
d) all of these