

Heat and Temperature
Heat is a measure of change, never a property possessed by an object or
system. Therefore, it is classified as a process variable. Temperature
describes the average kinetic energy of molecules within a material or system
and is measured in Celsius (°C), Kelvin(K), Fahrenheit (°F), or Rankine (R).
According to
the kinetic theory of particles, matter consists of tiny particles called…..
a) atoms
b) molecules
c) sub-atomic particles
d) ions
According to the kinetic theory of particles, atoms combine together to form…..
a) radical
b) molecules
c) sub-atomic particles
d) ions
Explanation:The kinetic theory of matter (particle theory) says that all matter consists of many, very small particles which are constantly moving or in a continual state of motion. The degree to which the particles move is determined by the amount of energy they have and their relationship to other particles
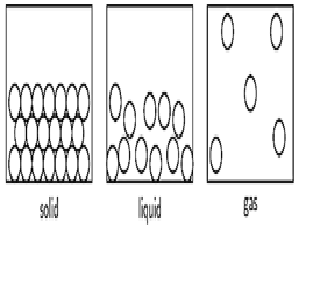
How many states
of matter present?
a) two
b) three
c) four
d) five
Explanation:1. Solid, 2. Liquid and 3. Gas
According to the kinetic theory of particles, particles which of the following state/s of matter only vibrate about their mean position?
a) solids
b) liquids
c) gases
d) all
According to the kinetic theory of particles, particles which of the following state/s of matter can flow?
a) solids
b) liquids
c) gases
d) both b and c