Some common examples of chemical reactions are………..
a) respiration
b) photosynthesis
c) burning
d) all of these
Those Substances which are produced as a result of chemical reaction are called…………
a) Electrons
b) Groups
c) Products
d) Reactants
Who proposed Law of conservation of mass?
(Mass can neither be created nor destroyed during a chemical reaction)
a) Antoine Lavoisier
b) Joseph Proust
c) Lorenzo Romano
d) Joseph Louis
Law of conservation of mass put forward in…
a) 1765
b) 1770
c) 1780
d) 1785
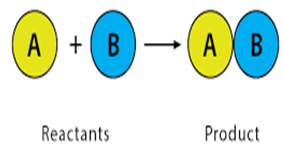
The chemical reactions occur where
two or more substance react together to form a one product is
called…………..reaction.
a) single displacement
b) synthesis
c) decomposition
d) none of these
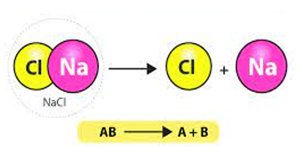
A
reaction in which a compound breaks down into two or more simpler
substances is called……..reaction.
a) single
displacement
b) synthesis
c) decomposition
d) none of these