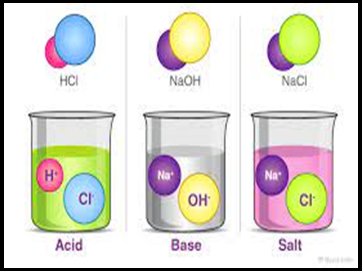
- There are three
main compounds of chemical (Acids, alkalis, salts).
- Properties of acids:
- They are sour in taste (the “acid” means sour).
- Acid are corrosive and can burn skin and dissolve
metal.
- Their aqueous solutions are good conductor
of electricity.
- They react with metals giving salt
and hydrogen gas.
- They turn the blue litmus paper red.
- They react with carbonates giving carbon dioxide gas.
- They react with alkalis giving a salt and water.
- Solution of acetic acid (CH3COOH) called “vinegar”
is used in many food preparation.
- Properties of Alkalis/Bases:\
- They have bitter taste.
- They are slippery to touch.
- Their aqueous solution is good conductor
of electricity.
- They react with non-metals giving a salt
and hydrogen gas.
- They turn the red litmus paper blue.
- They react with acids giving a salt and water.
- Alkalis are commonly use in soap.
- For the neutralization of acidity of stomach, “antacids”
are used, which contain some alkalis as their main ingredient.
- Properties of Salt:
- Salt dissolved readily in water.
- The aqueous solution of some salts are very good
conductor of electricity.
- They show no change of color on litmus paper.
- It is neutral in nature.
- The substance turn red when dipped in acid the pH is less
than 7 (acidic)
- Turn blue when dipped in alkalis the pH is greater than 7
(alkaline).
- Indicators Acid Alkali
- Litmus Red Blue
-
Methyl orange Pink Yellow
-
Phenolphthalein Colorless Deep pink
Which one of the following acids is
used in car batteries……………………..
a) HCl
b) HNO3
c) H2SO4
d)None of these
c
The color of red and blue litmus stays same in aqueous solution of…………..
a) HCl
b) HNO3
c) H2SO4
d) NaCl
d
The alkli used as an antacid is………………..
a) KOH
b) Ca(OH)2
c) NaOH
d) Al(OH)3
d
Which one of the following salts is used in the treatment of constipation……..
a) NaCl
b) MgSO4
c) CuSO4
d) NaHCO3
b
Rose petals turn blue in……………………..
a) Alkaline solution
b) Acidic solution
c) Salty solution
d)None of these
a


